What Causes Minerals To Very In Color
Color in Minerals
How do we perceive colour?
- colour perceived depends on the low-cal the object is viewed under: the issue of illumination type can exist very of import (fluorescent vs. incandescent light)
- human vision
- rods: low intensity low-cal -> one color perceived (gray)
- cones: three pigment types RGB, thus color is seen = %r + %grand +%b
- eyes most sensitive to green light.
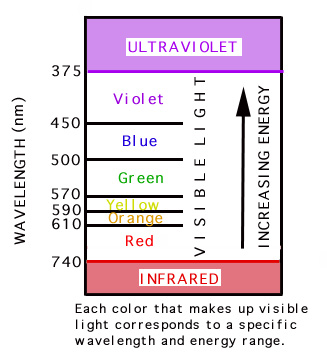
Electromagnetic spectrum
Why do things look colored?
Concrete processes
Causes of colour
Electromagnetic spectrum:
- Nosotros run across radiation with wavelengths in the "visible" spectrum
Visible spectrum: Red, Orangish, Yellowish, Greenish, Blue, Indigo, Violet.
Why do things expect colored?
- thing are colored when some procedure removes some wavelengths (absorbs specific wavelengths) from the visible spectrum
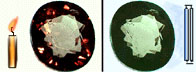
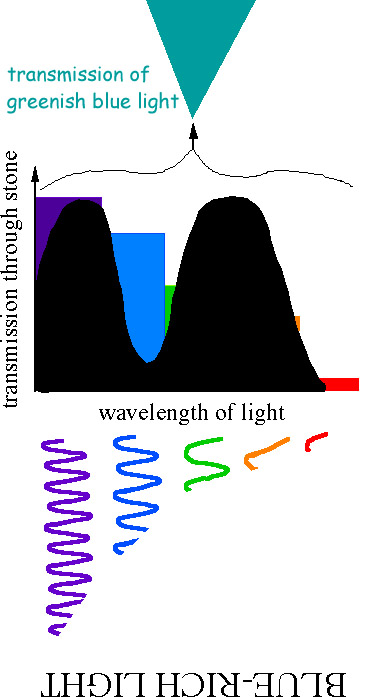
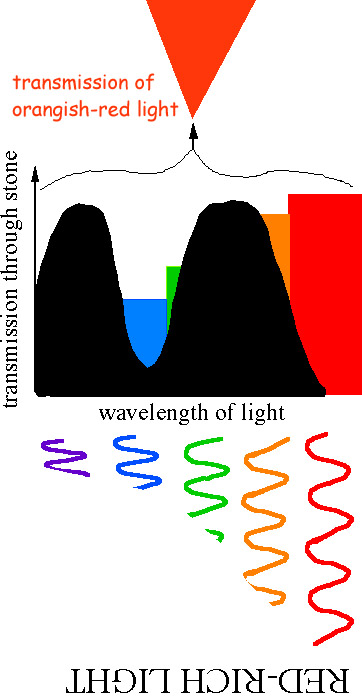
- Question: blueish sapphire viewed in candle looks black, why ?
- Blue sapphire is blueish because this is the simply wavelength range of visible light that can be transmitted by the stone. Candle light is rich in red wavelengths and poor in blue wavelengths. Thus, the wavelengths (colors) of visible light bachelor are exactly those that can NOT be transmitted. Outcome: no light is transmitted, the rock appears black!
If you empathize this, so you lot understand some of the important basic concepts in this module!
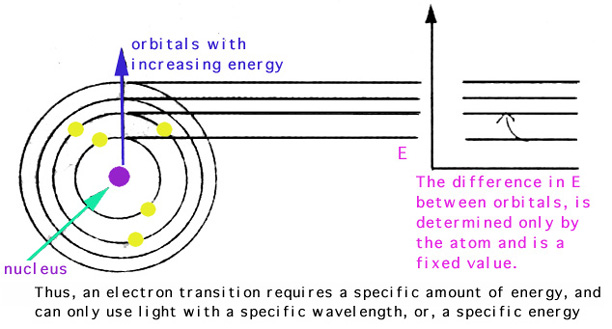
Concrete processes occurring in the stone:
An electron transition requires a specific corporeality of free energy, and tin only employ low-cal with a specific wavelength (each wavelength having a corresponding free energy).Allow's _define_ adsorbtion hither?
- luminescence: electron returns to its ground state (where it started) and releases the amount of energy it absorbed (thus returns light with that wavelength to the spectrum, thus, no color....
- fluorescence: If some of the adsorbed energy is lost but the reminder is returned to the visible spectrum, the light returned has lower energy and thus, dissimilar colour. [changes in free energy = changes in wavelength = changes in color].
Click for larger image!
Causes of colour in minerals
charge transfer
color centers
band theory (not required for EPS2)
physical optics (covered afterwards)
Impurities cause color in gems!
Impurities are elements (e.g., Ti, Five, Cr, Mn, Fe, Co, Ni, Cu...) that are not present in the pure compound. Impurities are elements that occur in depression concentration in the gemstone.Case:
A ruddy may contain < 1% Cr and information technology will await pink or red, but the aforementioned material without Cr volition be completely colorless. This example contrasts with gems such as turqoise, in which the color-causing impurity is a major ingredient.
If we have one mineral, beryl, and add together dissimilar impurities, we get different colors:
Beryl containing iron (Fe):
Beryl containing Manganese(Mn):
- Aquamarine = Fe++, beryl is blue
- Heliodor = Iron+++, yellow
- Dark-green beryl : due to mixtures of Fe2+ and Fe3+
Beryl containing Chromium(Cr):
- Morganite : Mn++ is pinkish
- Red beryl : Mn+++ is red
From the above examples information technology is articulate that the oxidation state (eastward.1000., Fe2+ vs. Fe3+) as well affects the color!
- Emerald = emerald greenish : Cr+++
If impurity ions produce color, the color can be changed if the oxidation state can be inverse.
Notation: heating beryl that is green or yellow reduces ferric iron, and the beryl turns blueish. This greatly increases the stone's value.
The process of color modify tin can merely involve heating the stone in a low oxygen atmosphere. This could be done by wrapping the rock in paper and allowing the newspaper to burn
Mn+++ is efficient at absorbing light, (blue end of the spectrum) thus colour is strong.
The same impurity colors dissimilar gems differently!
Example:Chromium (Cr+++) in ruby: red
Chromium (Cr+++) in beryl: emerald greenish
Chromium(Cr+++) in alexandrite: purplish or ruby (run into below!)
This consequence is because the Cr absorbs light differently when it is in beryl, emerald, and alexandrite. This is illustrated here for ruby, alexandrite and emerald
Note the unlike regions of absorption and transmission in the to a higher place diagram.
In the case of cherry-red, the largest valley (transmission window = low in the assimilation graph) occurs at the red finish of the spectrum, thus the stone substantially looks red. (However, a smaller transmission window may occur at blue wavelengths (as shown). This gives a purplish bandage to the blood-red color of ruddy).
In the case of emerald, well-nigh tramsmission occurs at green wavelengths and almost other wavelengths are absorbed strongly. Thus, emerald looks greenish.

The "Alexandrite" color change event:an instance where the details are important! Colour alter due to change in the color of incident light! (call up that fluorescent light is bluish (rich in blue wavelengths) and candle light is rich in reddish and orangish wavelenghts).Alexandrite is the all-time known example of a gemstone that changes color depending upon the low-cal it is viewed under.In the example of alexandrite, at that place are ii approximately equal sized tranmission windows - the get-go at bluish and second at red wavelengths. When viewed in lite made up of all wavelenghts, the stone tramsmits blue and red and ofttimes looks purple or majestic-grey.Here is a diagram showing the:instance of illumination of alexandrite with regular (white) liteWhen viewed in low-cal containing by and large red wavelengths (e.g., candle light) the stone looks carmine. This is understood because, although the rock could transmit blueish low-cal, there is no blue low-cal to transmit.
Here is a diagram showing illumination of alexandrite past reddish lite
The reverse is also true. In light rich in blue wavelengths (e.1000., fluorescent light), the stone looks blueish because, although it could also transmit red, at that place is little red in the light to transmit.
Hither is a diagram showing illumination of alexandrite by light rich in blue wavelenghts. Different specimens of the aforementioned gem will be characterized past slightly unlike adsorption/transmission characteristics (different adsorption spectra shapes) and so their colors will vary!Note: this color change effect in response to change in illumination type (e.g., incandescent vs. fluorescent) is not restricted to alexandrite! Many gems take color modify varieties, e.m., sapphire, garnet.colour change garnet viewed in fluorescent light color modify garnet viewed in incandescent light!
In all cases the explanation for color change is the same, involving the range of wavelengths in the light and the ability of the stone to transmit ii different ranges of wavelengths of light (east.g., crimson and green). Other examples.Visit a spectroscopy site with many additional examples of color caused by impurities!
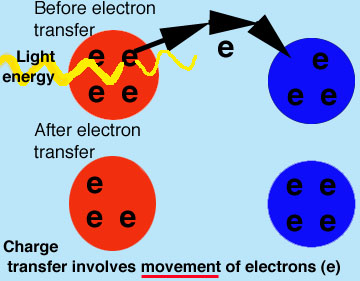
Accuse Transfer causes color in gems.
Charge transfer can but occur in compounds that have at least two elements in unlike and variable oxidation states. Charge transfer can produce very intense colors in gems and minerals.
The term accuse transfer refers to the process where electrons are swapped betwixt elements. Examples of elements that tin participate in charge transfer are:
- Fe2+ and Fe3+
- Ti3+ and Ti4+
- Mn2+ and Mn3+ and Mn4+ etc.
Furthermore, a crystal can contain mixtures of these elements (east.thou,. Mn and Atomic number 26) and these can participate in charge transfer.
Energy is absorbed from visible low-cal to transfer electrons from one atom to some other.
For example:A crystal contains metals (Thou) in 2 oxidation states: M2+ and M4+
- M2+ can loose an electron and become M3+
M4+ can accept the electron (from above) and become M3+.More examples: Visit a spectroscopy site with boosted information about color caused by charge transfer.
- Thus, the crystal tin can exist with
M3+ plus M3+ or M2+ plus M4+.Every bit you lot tin can see, these pairs are interchangeable by movement of an electron.
This is described more fully equally intervalence charge transfer!
Colour centers
Color centers are imperfections in crystals that crusade color (defects that cause color past absorption of low-cal).They are most oft due to radiation harm: e.chiliad., damage due to exposure to gamma rays. This irradiation may exist from both natural (U, Th, K in minerals) or artificial sources. In rare cases, UV low-cal can produce color centers.
If damaged by radioactive decay, electrons can be removed from their normal sites, bounciness around, loose energy, and somewhen come to residuum in a vacant site in the structure (a trap).
1 crystal may take many different types of electron traps
Electrons in specific traps blot only a sure range of wavelengths, color that is seen is the color non absorbed by these trapped electrons.
Examples:
Because they are a form of harm, color centers can exist removed past addition of energy. This may involve heating the rock to a few 100 C.
e.g,. light-green diamond: due to missing carbon cantlet which absorbs red light
- smoky quartz: Al+++ <=> Si4+
- Pure zircon is a colorless mineral, whereas zircon containing uranium (U) is blue and zircon damaged by radioactive decay of U is brownish-red!
Example: Heat care for dark-brown zircon, it may turn blueish!! (this is a common jewel treatment!)
Review: when electrons escape their traps, color centers are removed, so color is removed.
- In some cases, exposure to sunlight (especially UV) provides sufficient energy to remove the color heart! - amethyst is an instance.
We volition revisit this topic when nosotros hash out topaz, for case!
- Because irradiated minerals may have several color centers (several traps with different energies required to allow electrons to escape, color can be manipulated by selective removal of unwanted colour centers (controlled heating).
Visit a spectroscopy site with boosted examples of color caused by radiation impairment.
Other causes of color in minerals
Important for EPS2 students: Farther explanation of basic concepts.
Source: https://nature.berkeley.edu/classes/eps2/wisc/Lect7.html
Posted by: boykincasent.blogspot.com


0 Response to "What Causes Minerals To Very In Color"
Post a Comment